A new cancer treatment caused a woman’s tumor to virtually disappear in just 5 days
Last updated on
The Role of Collagen in Keeping Cancer Cells Dormant: A New Frontier in Cancer Treatment In the world of cancer treatment, progress often moves at a painstaking pace—measured in incremental survival gains, marginal response rates, and long-shot trials. So when news emerged that a woman’s aggressive brain tumor had nearly disappeared just five days after receiving a single dose of an experimental therapy, it sent ripples through the medical community. Not because it offered a miracle cure, but because it demonstrated, unequivocally, what science can achieve when innovation meets urgency.
This unprecedented outcome occurred at Massachusetts General Hospital, where researchers piloted a new form of CAR-T cell therapy against glioblastoma, one of the most lethal and treatment-resistant cancers known to medicine. The patient, a 57-year-old woman whose tumor had already defied standard treatments, experienced a rapid and near-total regression that challenged the field’s expectations. Though her remission was brief, the implications were profound: for the first time, a living medicine engineered from her own immune system had dismantled a glioblastoma in real time.
Glioblastoma — Medicine’s Most Formidable Adversary
Glioblastoma is widely regarded as one of the most devastating diagnoses in modern medicine, known for its aggressive behavior, complex biology, and tragically short survival outcomes. Affecting roughly 12,000 Americans each year, this malignant brain tumor is notorious not only for how quickly it grows, but for how persistently it returns despite the best available interventions. Standard treatments—surgical resection followed by radiation and chemotherapy—can sometimes slow its progression, but they rarely offer a lasting solution. Even when surgeons manage to remove large portions of the tumor, glioblastoma’s microscopic tendrils weave into healthy brain tissue, making complete eradication virtually impossible. Recurrence is not just common but expected, often more aggressive and treatment-resistant than before.
How Microplastics from ultra processed foods may harm your brain and mental health. A key reason for this resilience lies in the unique anatomical and biological environment of the brain. The blood-brain barrier, a protective filter that shields the brain from toxins and infections, also limits the entry of many therapeutic drugs, making systemic treatment far less effective than in other cancers. Radiation therapy, while targeted, cannot distinguish perfectly between malignant and healthy tissue, leading to collateral damage that impairs neurological function. Chemotherapy drugs face similar limitations and are often rendered ineffective by the tumor’s rapidly adapting biology. According to the American Brain Tumor Association, even with aggressive treatment, the median survival time for patients with recurrent glioblastoma remains around eight months. These harsh realities have forced oncologists and researchers to look beyond conventional tools, seeking entirely new strategies to outmaneuver a disease that has long outpaced them.
What makes glioblastoma especially challenging is its ability to evolve in real time. Unlike some cancers that follow predictable patterns, glioblastoma tumors are highly heterogeneous, composed of diverse cell populations that express different proteins and genetic mutations. Treatments that target a single molecular pathway often succeed briefly before resistant cells take over. As Dr. Bryan Choi of Massachusetts General Hospital explained, “Tumors often come back more aggressive and more resistant after each round of therapy.” In this context, traditional treatment approaches are increasingly viewed as stopgaps rather than solutions. The desperate need for new, adaptable, and more precise therapies has led scientists to explore immunotherapy and gene editing as tools that might finally shift the odds in patients’ favor. It is within this landscape of persistent failure and scientific urgency that a revolutionary new treatment has emerged—one that could redefine the boundaries of what’s possible in brain cancer care.
Engineering the Immune System — How CAR-T Therapy Is Being Reimagined for Brain Cancer
Chimeric Antigen Receptor T-cell therapy, or CAR-T, represents one of the most significant breakthroughs in cancer medicine over the past decade. Originally developed for blood cancers like leukemia and lymphoma, this approach engineers a patient’s own immune cells to identify and destroy cancer cells with a level of precision that traditional therapies cannot match. The process begins with collecting T cells—white blood cells essential to immune defense—through a procedure called leukapheresis. These cells are then modified in a laboratory using viral vectors that insert new genetic instructions, enabling them to recognize specific markers on cancer cells. Once reprogrammed, the T cells are expanded, tested for safety and potency, and then reintroduced into the patient’s body to seek and destroy malignant cells. The results in hematologic cancers have been nothing short of revolutionary, leading to durable remissions in patients who had exhausted every other option.
However, extending CAR-T’s success to solid tumors like glioblastoma has proven far more complex. Blood cancers typically involve more uniform targets, making them easier for engineered cells to identify. Solid tumors, on the other hand, are highly heterogeneous, meaning their cells display a variety of markers that can change over time. Additionally, the tumor microenvironment in organs like the brain can suppress immune responses and block the infiltration of therapeutic cells. Researchers have long been aware that a single-target CAR-T approach was unlikely to succeed against such adaptable and fortified foes. What was needed was a new kind of immune cell—one capable of multitasking, recognizing multiple tumor markers, and rallying other components of the immune system to assist in the fight.
Massachusetts General Hospital developed such a solution with CARv3-TEAM-E cells, a novel therapy designed specifically to target glioblastoma’s most elusive traits. These engineered cells go after EGFRvIII, a mutated protein commonly found in glioblastoma, while simultaneously releasing antibodies that target wild-type EGFR proteins expressed on other tumor cells. In essence, they create a multi-pronged assault, attacking not just one, but several features of the tumor at once. This innovation also includes a mechanism to recruit the patient’s broader immune response, turning the therapy into a collaborative effort rather than a solitary intervention. Administered directly into the brain’s ventricular system through a surgically implanted reservoir, these CAR-T cells bypass the blood-brain barrier, accessing cancer sites that traditional drugs cannot reach. It’s a sophisticated, highly localized strategy that addresses many of the limitations that plagued earlier attempts to use immunotherapy for brain tumors.
The level of customization and precision involved in developing CARv3-TEAM-E is a testament to how far cancer treatment has evolved from one-size-fits-all approaches. Each patient’s therapy is individually manufactured, a process that takes several weeks of meticulous laboratory work. From cell harvesting to genetic reprogramming and quality control, the entire journey reflects a shift in oncology—from treating cancer as a uniform disease to addressing it as a dynamic, evolving adversary that requires tailored solutions. By combining insights from immunology, molecular biology, and neurosurgery, researchers are forging a new path forward—one where the immune system is no longer a bystander in brain cancer but a central force in its defeat.

Image source: The New England Journal of Medicine. DOI: 10.1056/NEJMoa231439
A Five-Day Miracle — Inside One Patient’s Extraordinary Response
Among the three patients who received the CARv3-TEAM-E therapy during the INCIPIENT clinical trial, one woman’s case stood out—not just for the speed of her tumor’s response, but for how radically it challenged what medicine has come to expect from glioblastoma. At 57, she had already endured six months of grueling treatment. Her cancer had returned despite surgery, radiation, and chemotherapy. When she entered the trial at Massachusetts General Hospital, expectations were cautious. Doctors hoped for stabilization, maybe modest shrinkage. But what happened within five days of her receiving a single dose of engineered T cells startled even seasoned researchers. MRI scans taken just 120 hours after the infusion revealed that her previously unyielding tumor had nearly vanished.
The infusion itself was delivered directly into her brain via an Ommaya reservoir—a small, surgically implanted port that allows access to the ventricles where cerebrospinal fluid flows. This method ensured that the modified T cells reached the tumor’s location without being filtered or diluted by other parts of the body. As expected, the patient experienced inflammation-related side effects—fever, fatigue, and confusion—but these were managed and largely subsided by day four. What remained was a transformation few thought possible: from visible, active tumor to near-complete regression in less than a week. It was a striking display of how quickly and effectively the immune system, when properly equipped, could dismantle even the most entrenched cancer.
Yet the story is both inspiring and cautionary. While the initial results were astonishing, the tumor eventually returned within a month. This pattern, seen in two of the three trial participants, illustrates one of the therapy’s current limitations—durability. The modified T cells peaked in activity around day 21, but then declined. Without sustained immune surveillance, the surviving cancer cells regrouped and began rebuilding. Still, the woman’s five-day response demonstrated something profound: that glioblastoma, long considered intractable, could be rapidly dismantled by a well-designed immune attack. It proved that even in the most hostile terrain, immunotherapy could deliver a blow strong enough to reset what’s considered medically possible.
Liquid biopsies supported these clinical observations. Before treatment, the patient’s cerebrospinal fluid showed high levels of cancer DNA associated with EGFRvIII and wild-type EGFR mutations. Within days of receiving the therapy, those genetic markers dropped to undetectable levels. The modified cells had done their job—targeting and eliminating the malignant cells bearing those signatures. Although the remission was short-lived, the magnitude and speed of the response signaled that this line of therapy held genuine, if still imperfect, promise. For a disease that rarely offers even temporary reprieve, five days of near-complete regression isn’t just a medical curiosity—it’s a potential turning point.
Image source: MRI in Participant 3, INCIPIENT trial. Image courtesy of The New England Journal of Medicine.
Building the Future — Scientific Collaboration, Challenges, and Next Steps
Behind this dramatic clinical success is a larger story of institutional collaboration, scientific discipline, and a commitment to accelerating research without compromising safety. The CARv3-TEAM-E therapy didn’t emerge from a vacuum; it was the product of years of meticulous work led by Dr. Marcela Maus and a multidisciplinary team at Massachusetts General Hospital. Their approach exemplified what’s often described as “bench-to-bedside” innovation—translating discoveries made in research labs into treatments tested in human patients. Achieving this in under five years is remarkable in the world of medicine, where new therapies typically require a decade or more to reach clinical trials.
This speed was made possible by institutional infrastructure such as the Connell and O’Reilly Families Cell Manipulation Core Facility and the Gene and Cell Therapy Institute, which provided the tools and regulatory support to produce these therapies under rigorous quality standards. Neurosurgeons, oncologists, immunologists, and molecular biologists worked in tandem to oversee every phase—from cell engineering and manufacturing to patient monitoring and data collection. Their ability to coordinate across departments, coupled with strong backing from the hospital’s leadership, underscores how essential interdisciplinary collaboration is to achieving true innovation in medicine.
Even so, the path forward remains filled with scientific and logistical hurdles. Chief among them is extending the duration of therapeutic effects. Researchers are already investigating methods to make CAR-T cells more persistent in the brain, such as repeat infusions timed strategically to maintain immune pressure or preconditioning regimens that reduce immune rejection of the modified cells. There’s also interest in combining CAR-T with checkpoint inhibitors or cancer vaccines to create a multi-front attack. The complexity of glioblastoma demands such layered strategies, as monotherapies, no matter how advanced, are unlikely to hold the line indefinitely.
Moreover, questions of scalability and access loom large. Personalized therapies like CAR-T are resource-intensive, requiring advanced lab capabilities and skilled personnel. Bringing these treatments to a broader population will necessitate innovations not just in science but in healthcare logistics, policy, and economics. Still, the proof-of-concept is in hand: when properly designed, a patient’s own immune system can be weaponized to dismantle even the most intractable tumors. That realization has already begun to reshape the landscape of oncology research.
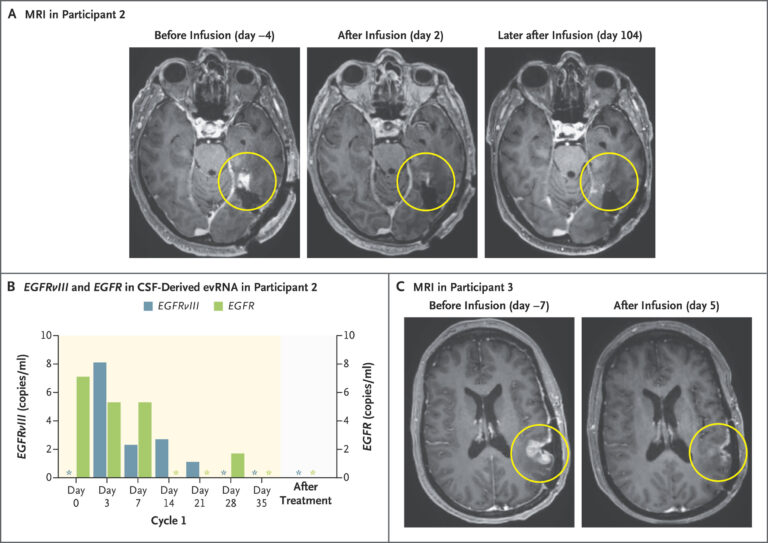
Image courtesy of The New England Journal of Medicine.
Toward a New Era — Hope, Caution, and Urgency
The story of a tumor that nearly vanished in five days is not just a scientific milestone—it is a call to action. It reminds us that even the most devastating diseases can yield to persistence, ingenuity, and cross-disciplinary collaboration. While the trial results do not yet represent a cure, they point clearly toward a future in which brain cancer is no longer synonymous with hopelessness. This is not a promise of miracles, but of momentum—hard-won, evidence-based progress that deserves to be nurtured and expanded.
For patients and families affected by glioblastoma, the ability to see measurable regression within days of a new therapy is profoundly meaningful, even if temporary. It opens the door to imagining what might be possible with further refinement—treatments that don’t just delay progression, but deliver lasting remissions. For scientists and clinicians, the trial offers critical insights into how immune cells behave in the brain, what causes therapeutic decline, and how future protocols can be improved. Every data point from these patients serves as a foundation for the next iteration of therapy.

But the speed at which this trial was launched also highlights a deeper urgency. Cancer doesn’t wait, and neither should innovation. Regulatory frameworks, funding models, and research institutions must evolve to support rapid yet responsible development of high-risk, high-reward therapies. Massachusetts General’s achievement illustrates what can happen when the right resources align with scientific clarity and clinical need. The next phase—larger trials, refined protocols, and broader applications—requires sustained commitment from all corners of the medical ecosystem.
In the end, the five-day response of one patient may be fleeting, but its impact is enduring. It proves that glioblastoma, long seen as invincible, can be shaken at its core. Now the challenge is to transform momentary regression into lasting remission. If medicine can meet that challenge, we may soon find ourselves not just treating cancer, but outsmarting it.
Some of the links I post on this site are affiliate links. If you go through them to make a purchase, I will earn a small commission (at no additional cost to you). However, note that I’m recommending these products because of their quality and that I have good experience using them, not because of the commission to be made.































 JOIN OVER
JOIN OVER
Comments