A Gene Targeted Treatment Marks a Potential Breakthrough in ALS Research
Last updated on
For decades, a diagnosis of amyotrophic lateral sclerosis has carried with it an almost unbearable certainty. Patients and families are told to prepare for relentless decline, loss of independence, and a future measured not in years, but often in months. Treatments approved over the years have offered only modest extensions of life, with effects so subtle that many patients never feel a tangible difference. That is why a new study examining the drug tofersen is capturing attention across the ALS community and beyond. For the first time in modern ALS research, doctors are documenting something that was long thought impossible for this disease: not just slowing progression, but in some patients, stabilization and even partial recovery of lost function. The findings, published in the medical journal JAMA, focus on a rare genetic form of ALS affecting a small percentage of patients. While the drug is not a cure and will not help everyone with ALS, researchers and clinicians say it represents a profound shift in how the disease might be treated in the future.Understanding Als and Why Progress Has Been So Difficult
Amyotrophic lateral sclerosis, often referred to as ALS or Lou Gehrig’s disease, is a progressive neurodegenerative disorder that affects motor neurons in the brain and spinal cord. These nerve cells are responsible for controlling voluntary muscle movement, including walking, speaking, swallowing, and breathing. As motor neurons deteriorate, they stop sending signals to muscles. Without these signals, muscles weaken, shrink, and eventually stop functioning altogether. Over time, patients lose the ability to perform basic daily activities and ultimately require assistance with breathing. According to the National Institute of Neurological Disorders and Stroke, ALS is relentlessly progressive, meaning symptoms worsen over time. There is currently no known cure, and the average life expectancy after diagnosis is typically two to five years, though some patients live longer. One of the greatest challenges in developing ALS treatments is the disease’s complexity. About 90 percent of cases are considered sporadic, with no clearly identifiable cause. The remaining cases are familial, linked to inherited genetic mutations. Even within these genetic forms, the mechanisms that drive neuron death can vary significantly. For years, researchers have tested drug after drug, many of which showed promise in animal models but failed to deliver meaningful benefits in humans. As a result, ALS research has been marked by frustration, dashed hopes, and incremental gains at best.A Drug Designed for a Specific Genetic Mutation
What the Latest Study Found

Patient Stories That Are Reshaping Expectations
Jessica Morris, a 37 year old social worker and mother of three, began experiencing ALS symptoms in early 2022 when her left knee started buckling due to muscle weakness. Within months, her condition worsened rapidly, and by late 2022 she relied on a wheelchair for daily activities. Morris carries a SOD1 mutation but was diagnosed after the tofersen trial had already begun. Through the FDA’s expanded access pathway, she was allowed to receive the drug outside of the clinical trial. After starting treatment in December 2022, she noticed gradual changes. A few months later, something extraordinary happened. One evening, as she approached the stairs in her home, her body simply took a step without conscious effort. For the first time in months, she was walking upstairs. Over time, Morris found herself relying less on her wheelchair. Eventually, she no longer needed it at all and transitioned to using a cane. For her, the drug did more than slow progression. It restored a level of independence she thought was permanently lost. Another patient, Todd Legg, entered the clinical trial in 2020 after a rapid and frightening decline. His arms were weakening, his breathing was deteriorating, and his stamina had plummeted. Within two years of starting tofersen, his disease trajectory changed dramatically. His breathing stabilized, his legs remained strong, and his overall decline slowed to a pace his doctors described as extraordinary. Clinicians involved in the trial have reported similar experiences with other patients. While not everyone responds to the same degree, a subset of patients appear to experience a plateau in disease progression that challenges long held assumptions about ALS.A new drug may slow progression of — and even reverse — symptoms of a rare form of amyotrophic lateral sclerosis, or ALS, a new study found. https://t.co/w7hj1T9cmh
— ABC News (@ABC) December 25, 2025
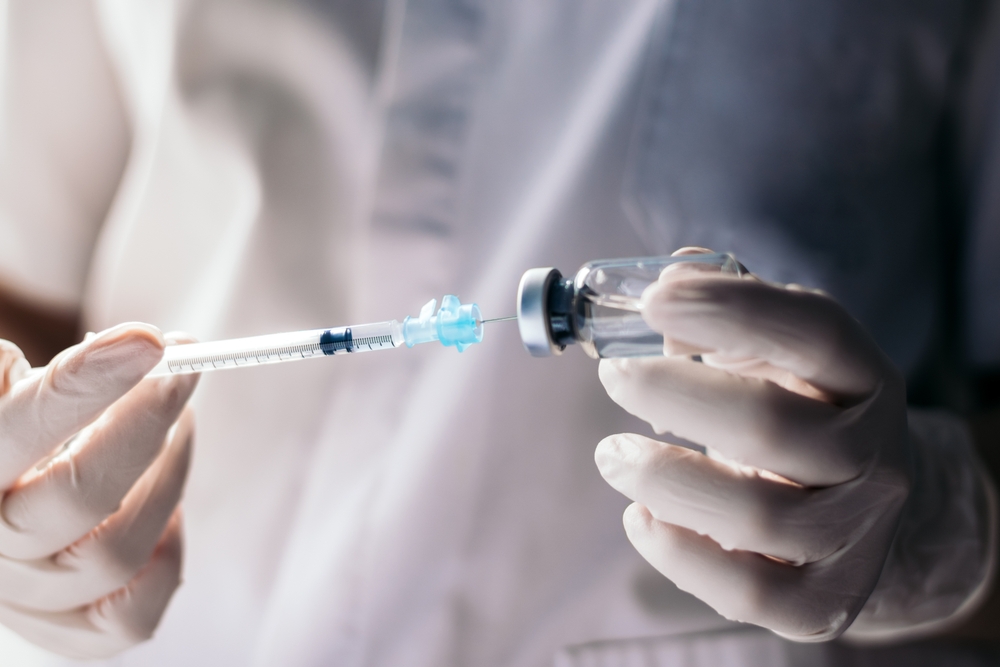
How Tofersen is Administered and Monitored
Why This Matters Even for Patients Who Cannot Benefit

The Growing Impact of Als in an Aging Population
The urgency of these advances is underscored by changing demographics. The Centers for Disease Control and Prevention estimates that approximately 34,000 people in the United States are currently living with ALS. Projections suggest that this number will rise by more than 10 percent by 2030, reaching over 36,000 cases. The largest increase is expected among individuals aged 66 and older, with a projected rise of 25 percent compared to 2022 levels. As the population ages, the burden of neurodegenerative diseases like ALS is expected to grow. Treatments that slow progression, preserve independence, and extend meaningful quality of life could have profound implications not only for patients, but also for caregivers and healthcare systems.
Ongoing Trials and the Future of Gene Targeted Therapy
The story of tofersen is still unfolding. Researchers are currently conducting additional studies to explore whether the drug can be even more effective when given earlier. One such study is the ATLAS trial, which is enrolling individuals who carry SOD1 mutations but have not yet developed symptoms of ALS. The goal is to determine whether starting tofersen before symptoms appear can delay or prevent the onset of the disease altogether. If successful, this approach would represent a dramatic shift in ALS care, moving from reactive treatment to proactive prevention for at risk individuals. At the same time, scientists are investigating antisense therapies for other neurodegenerative diseases, including Huntington’s disease and certain forms of dementia. The success of tofersen strengthens confidence that these strategies can translate from the lab to real world benefit.Balancing Hope With Realism
Despite the excitement, researchers and clinicians emphasize the importance of cautious optimism. Tofersen is not a cure, and it does not work equally well for everyone. Some patients experience only modest slowing of disease progression, while others see more dramatic benefits. There are also practical challenges. Genetic testing is required to identify eligible patients, and access to specialized care is not uniform across regions. The cost and long term logistics of monthly intrathecal injections must also be considered. Still, for a disease long defined by therapeutic failure, even partial success represents a monumental shift.A new chapter in ALS research
For patients like Jessica Morris, Todd Legg, and Rickey Malloy, tofersen has already changed the course of their lives. It has given them time, independence, and a sense of possibility that once seemed unimaginable. For the broader ALS community, the drug represents something equally powerful: evidence that the disease is not invincible. By unraveling its genetic underpinnings and targeting them with precision, researchers are beginning to rewrite the narrative of ALS. While only a small group of patients can benefit today, the lessons learned from tofersen are likely to shape the future of ALS treatment for years to come. In a field where hope has often been fleeting, this breakthrough offers something rare and precious: a reason to believe that progress, once elusive, is finally within reach.Some of the links I post on this site are affiliate links. If you go through them to make a purchase, I will earn a small commission (at no additional cost to you). However, note that I’m recommending these products because of their quality and that I have good experience using them, not because of the commission to be made.































 JOIN OVER
JOIN OVER
Comments