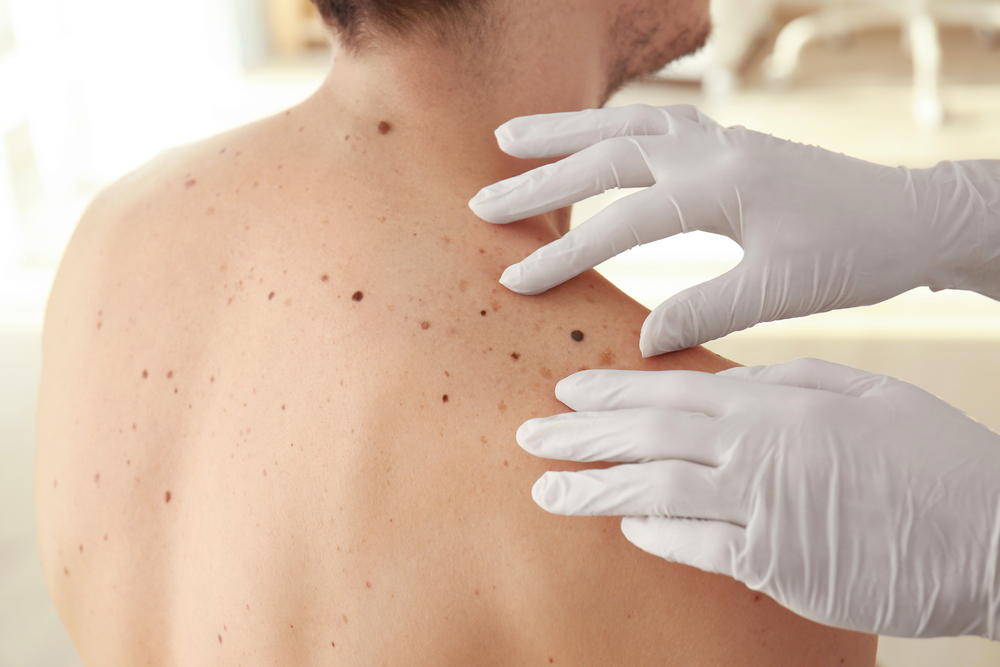
Modified Herpes Virus Shrinks Advanced Melanoma Tumors in Trials, Offering Fresh Hope Against Stubborn Skin Cancer
Last updated on
Everyday viruses are often seen as unwelcome guests causing cold sores, fevers, or worse. But what if one of them could be repurposed as a life-saving ally? The herpes simplex virus type 1, best known for triggering cold sores, has long carried a social and medical stigma. Yet in a twist few would have predicted, scientists have turned this common virus into a promising cancer fighter. By reengineering its genetic code, researchers have created a version of herpes that not only infiltrates deadly tumors but rallies the body’s immune system to finish the job. This approach isn’t science fiction it’s happening in clinical trials right now. In patients with advanced melanoma, a notoriously aggressive and treatment-resistant form of skin cancer, the virus-based therapy is showing measurable results. Tumors are shrinking. In some cases, they’re vanishing entirely. With nearly half of all advanced melanoma cases failing to respond to existing immunotherapies, the need for innovative treatments has never been more urgent. Could a virus we’ve long tried to suppress become the very tool that helps us fight one of cancer’s deadliest forms? The answer, it seems, is beginning to emerge.Turning a Nuisance Virus Into a Cancer Fighter
Herpes simplex virus type 1 (HSV-1) has long been known for its irritating legacy: cold sores, fever blisters, and, in rare cases, severe illness in immunocompromised individuals. But through decades of research and precise genetic engineering, this once-problematic virus is being repurposed into a tool of modern medicine one capable of targeting and destroying cancer. At the heart of this breakthrough is a growing field called oncolytic virotherapy, which uses genetically modified viruses to infect, replicate within, and ultimately kill cancer cells. HSV-1 offers a unique advantage: its large DNA genome provides researchers with the flexibility to delete harmful genes and insert new therapeutic ones. This allows scientists to strip the virus of its disease-causing abilities while enhancing its capacity to target tumors and activate the immune system. Researchers like Prof. Susanne Bailer, a virologist at Germany’s Fraunhofer Institute, have played a key role in refining these techniques. Her team developed a version of HSV-1 that includes genetic “safety switches” to ensure the virus only multiplies within cancerous cells not healthy ones. Once inside the tumor, the virus replicates and causes cancer cells to rupture, a process known as lysis. This destruction releases tumor-specific markers, which alert the body’s immune system to the presence of cancer effectively turning a previously evasive enemy into a visible target.In addition to releasing these markers, the modified virus can be engineered to secrete proteins that further activate immune responses. This dual action direct tumor destruction and immune activation makes oncolytic viruses a powerful candidate for treating cancers that have developed resistance to conventional therapies. The concept of weaponizing viruses against cancer isn’t new. As early as the early 1900s, doctors noted that certain viral infections coincided with unexpected tumor regression. But only in the last three decades have advances in molecular biology and gene editing made it possible to manipulate viruses with the precision needed for cancer treatment. What makes HSV-1 particularly promising is its track record. Unlike newer, lesser-known viruses, herpes has been extensively studied for decades. Its behavior, lifecycle, and interaction with the human body are well understood, making it a manageable and modifiable platform. Additionally, the availability of long-established antiviral drugs provides a reliable fail-safe if unintended side effects occur.The virus we usually avoid is now saving lives. A @KeckMedicineUSC trial found that a modified herpes virus shrank melanoma tumors in one-third of advanced cases. https://t.co/8RAJpOJSeC
— USC (@USC) July 14, 2025
How the Therapy Works: Inside the Science of RP1


What the Trials Are Showing

A New Chapter in Melanoma Treatment
The Future of Virus-Based Therapies
A New Era of Immunotherapy Takes Shape
The idea that a virus long associated with cold sores could become a cornerstone in cancer treatment is as improbable as it is inspiring. Yet the progress made in clinical trials with RP1 a genetically modified herpes virus is undeniable. For patients with advanced melanoma who have exhausted traditional therapies, this approach offers something increasingly rare in the world of late-stage cancer: hope backed by data. The results so far signal more than just a medical milestone; they represent a new way of thinking about cancer therapy. Rather than relying solely on toxic, broad-spectrum treatments, researchers are engineering smarter, more targeted solutions that harness the body’s own immune system turning it from passive observer to active defender. What makes this approach especially promising is not only its effectiveness but also its safety and adaptability. Virus-based therapies like RP1 are well tolerated, customizable, and increasingly supported by regulatory bodies like the FDA. For a disease like melanoma, where resistance to existing treatments is common and often devastating, this innovation marks a meaningful shift in the therapeutic landscape. But the impact doesn’t end with melanoma. The same foundational science behind RP1 is now being applied to other hard-to-treat cancers from lung to pancreatic and holds the potential to revolutionize how we approach tumors that have, until now, defied even the most aggressive therapies. Still, this is just the beginning. Ongoing Phase 3 trials, broader patient access, and continued research into combination therapies will be crucial in determining how far virus-based treatments can go. If the current trajectory holds, oncolytic viruses may soon be a routine part of how we fight cancer not as miracles, but as carefully developed tools shaped by decades of scientific persistence. For patients, families, and researchers alike, this moment is not just about shrinking tumors. It’s about reclaiming possibilities where none existed. It’s a reminder that even the most unlikely agents of harm can be transformed into instruments of healing and that innovation, when grounded in science, can change lives.Some of the links I post on this site are affiliate links. If you go through them to make a purchase, I will earn a small commission (at no additional cost to you). However, note that I’m recommending these products because of their quality and that I have good experience using them, not because of the commission to be made.




























 JOIN OVER
JOIN OVER
Comments