A Universal Immune Therapy May Change Breast Cancer Treatment Forever
Last updated on
For decades, cancer treatment has followed a familiar pattern. First comes diagnosis, then a combination of surgery, radiation, chemotherapy, or targeted drugs, often customized to the molecular signature of a patient’s tumor. While this approach has saved many lives, it has also revealed a hard truth. Cancer, especially solid tumors like breast cancer, is remarkably adaptable. It evolves, resists, and frequently returns in new forms that evade even the most advanced therapies. In recent years, immunotherapy has offered a radically different strategy. Instead of attacking cancer directly with chemicals or radiation, immunotherapy aims to empower the body’s own immune system to recognize and destroy malignant cells. This approach has already transformed the treatment of certain blood cancers. But solid tumors have remained a far greater challenge. Now, researchers have unveiled a new type of immune cell therapy that could mark a turning point. Scientists have developed what they describe as a one-product-fits-all immunotherapy for breast cancer, a treatment that can be mass-produced, stored, and used immediately rather than custom-built for each patient. Even more striking, this therapy appears capable of attacking tumors from multiple angles at once, making it far harder for cancer to escape. This breakthrough centers on a powerful and relatively rare immune cell known as the invariant natural killer T cell, or NKT cell. When engineered with a chimeric antigen receptor, the resulting CAR-NKT cells may finally overcome many of the obstacles that have limited immunotherapy in solid tumors.Why Breast Cancer Has Been So Difficult for Immunotherapy
Breast cancer is one of the most common cancers worldwide and remains a leading cause of cancer-related death. Despite major advances in early detection and treatment, certain subtypes, especially triple-negative breast cancer, have limited therapeutic options and poor outcomes once they spread beyond the breast. One reason immunotherapy has struggled in breast cancer is that many tumors are considered immunologically cold. This means they do not naturally attract large numbers of immune cells, nor do they present strong signals that alert the immune system to danger. Instead, they actively suppress immune activity in their surroundings. The tumor microenvironment plays a central role in this suppression. Solid tumors reshape their local environment in ways that exhaust immune cells and blunt their killing ability. Oxygen levels drop, acidity increases, and nutrients become scarce. Tumors also release molecules that actively turn off immune responses, including cytokines like transforming growth factor beta and metabolites such as adenosine. As a result, even immune cells that are capable of killing cancer often become dysfunctional once they enter the tumor. They lose activating receptors, slow their metabolism, and eventually enter a state of exhaustion. This hostile environment has been one of the main reasons why CAR-T cell therapies, so successful in blood cancers, have shown limited effectiveness in solid tumors like breast cancer.The Promise and Limits of CAR-T Therapy

Enter Natural Killer Cells and NKT Cells
Engineering a Multipronged Attack

A Universal, Off-the-Shelf Therapy
Evidence from Advanced Breast Cancer Models
In preclinical studies, CAR-NKT cells were tested against tumor samples from patients with late-stage metastatic breast cancer. The results were striking. The engineered cells successfully killed cancer cells in every sample tested. Just as importantly, they also eliminated the immunosuppressive cells that tumors use as protective escorts. This dual action suggests that CAR-NKT therapy does more than simply attack cancer cells. It actively dismantles the environment that allows tumors to survive and spread. The therapy showed particular promise in triple-negative breast cancer, a subtype that lacks hormone receptors and HER2 expression, making it resistant to many targeted therapies. For patients with this diagnosis, treatment options are often limited to chemotherapy, with modest survival benefits. By contrast, CAR-NKT therapy demonstrated robust activity across diverse tumor samples, hinting at a level of versatility rarely seen in cancer treatment.
Beyond Breast Cancer: A Platform Technology
While breast cancer has been the initial focus, the implications of CAR-NKT therapy extend far beyond a single disease. Mesothelin is overexpressed in several other solid tumors, including pancreatic, ovarian, and lung cancers. These malignancies are among the deadliest, in part because they resist most existing treatments and are often diagnosed at advanced stages. Preclinical studies have already shown that CAR-NKT cells can track and destroy metastatic pancreatic tumors, even after they have spread to distant organs like the liver and lungs. This ability to home in on metastases addresses one of the most stubborn challenges in oncology. Because the therapy targets both cancer cells and the suppressive microenvironment, it may remain effective even as tumors evolve and change their molecular identity. Researchers describe this approach as a platform technology. By swapping out the CAR target, the same NKT cell backbone could potentially be adapted to treat many different cancers.Rethinking the Tumor Microenvironment
One of the deeper lessons emerging from this research is the importance of the tumor microenvironment itself. For years, cancer treatment focused primarily on killing tumor cells directly. But it has become increasingly clear that the surrounding environment often determines whether a therapy succeeds or fails. Hypoxia, acidic conditions, metabolic competition, and immunosuppressive signaling all work together to neutralize immune attacks. Therapies that ignore these factors frequently show impressive results in the lab, only to fail in patients. CAR-NKT therapy stands out because it actively engages with this environment rather than trying to bypass it. By eliminating suppressive immune cells and resisting exhaustion, the engineered cells reshape the battlefield in their favor. This shift reflects a broader trend in cancer research, one that views tumors not as isolated masses of malignant cells but as complex ecosystems that must be disrupted at multiple levels.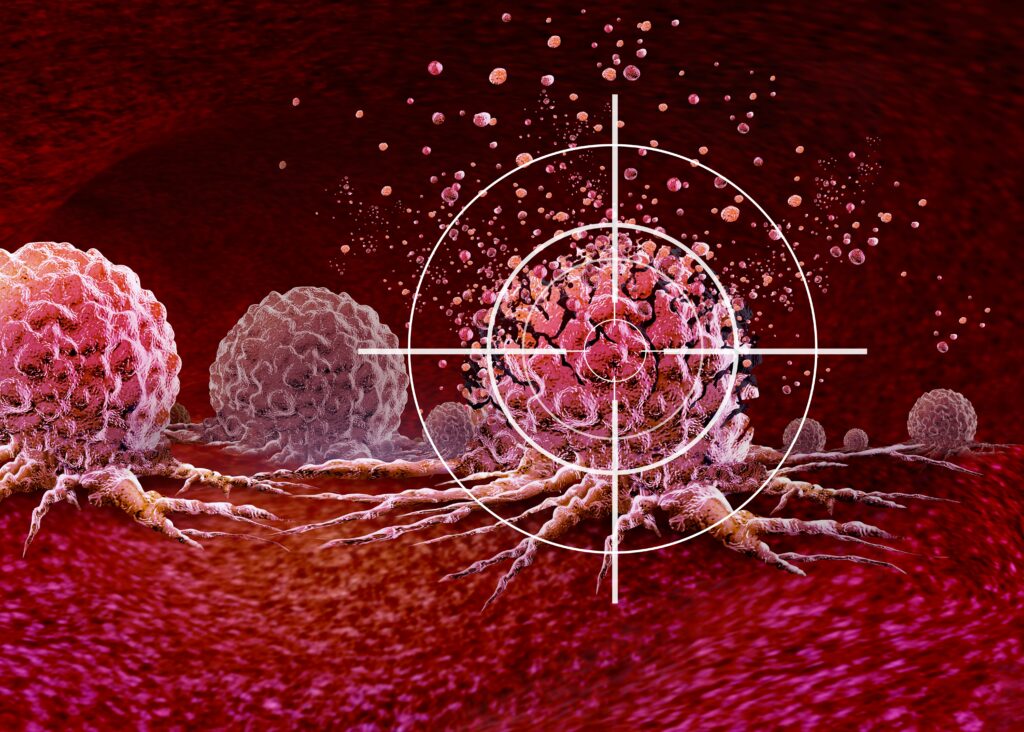
Safety and the Path to Clinical Trials
Safety is always a central concern with immune-based therapies. Excessive immune activation can be just as dangerous as cancer itself. So far, CAR-NKT cells have shown a favorable safety profile in preclinical studies. Their limited lifespan in the body reduces the risk of long-term off-target effects, and their innate regulatory mechanisms help prevent runaway inflammation. Researchers are now preparing regulatory applications to begin clinical trials in humans. These trials will be critical for confirming not only effectiveness but also safety, dosing, and durability of response. If early results translate to patients, CAR-NKT therapy could represent one of the most significant advances in solid tumor immunotherapy to date.A Glimpse into the Future of Cancer Treatment
The development of a one-product-fits-all immunotherapy for breast cancer challenges many long-held assumptions in oncology. It suggests that personalized medicine does not always have to mean individualized manufacturing. Sometimes, the key lies in finding immune strategies that are naturally adaptable. This research also highlights the growing sophistication of immunoengineering. Scientists are no longer simply boosting the immune system. They are rewiring it, equipping cells with new senses, new responses, and new resilience. At a deeper level, this work reflects a shift in how we understand disease. Cancer is no longer seen solely as a genetic malfunction within cells, but as a breakdown in communication, recognition, and balance within the body. By restoring those processes, therapies like CAR-NKT cells hint at a future where cancer treatment is not only more effective, but also more harmonious with the body’s natural defenses. For patients facing aggressive breast cancer and other solid tumors, that future cannot come soon enough. <!– /wp:paragraphSome of the links I post on this site are affiliate links. If you go through them to make a purchase, I will earn a small commission (at no additional cost to you). However, note that I’m recommending these products because of their quality and that I have good experience using them, not because of the commission to be made.




























 JOIN OVER
JOIN OVER
Comments