A New Nasal Spray Reverses Memory Loss in Alzheimer’s Mice, Study Finds
Last updated on
Something unusual happened in a Japanese laboratory when mice with Alzheimer’s disease started remembering again. Scientists at the Okinawa Institute of Science and Technology have developed a synthetic peptide that reversed cognitive decline in mouse models of Alzheimer’s disease. No human trials have taken place yet, and years of testing remain before patients could access such a treatment. But early results from mouse studies, published in Brain Research, suggest researchers may have found a way to rescue brain function before permanent damage sets in. Alzheimer’s disease has long resisted treatment. By the time symptoms appear, the brain has often deteriorated beyond repair. Most therapies focus on slowing decline rather than reversing it. Yet a team led by Professor Emeritus Tomoyuki Takahashi has taken a different approach, targeting the disease at a molecular level that scientists had previously overlooked. Their findings raise a tantalizing question. What if memory loss could be undone?A Disease That Defies Treatment
Synapses Under Siege
A Peptide Called PHDP5

Why a Nasal Spray?
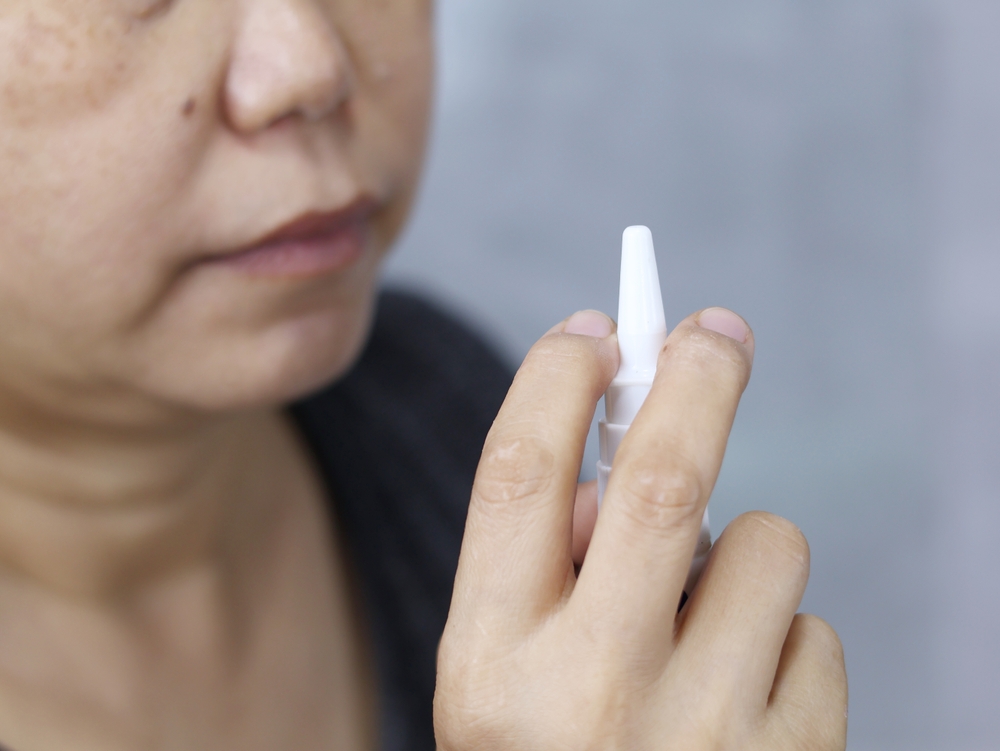
Mice That Remembered Again
Alzheimer’s Breakthrough: New Peptide Treatment Reverses Cognitive DeclineResearchers tested PHDP5 on two different mouse models of Alzheimer’s disease. Tau609 mice carry a human tau gene mutation and develop memory problems around six months of age. 3xTg-AD mice carry three different mutations associated with Alzheimer’s and show similar cognitive decline. After four weeks of treatment via nasal spray, mice underwent testing in a Morris Water Maze. Animals were placed in a pool of water and trained to find a hidden platform using visual cues around the room. Healthy mice learn the platform’s location quickly and remember it well. Mice with Alzheimer’s struggle with both learning and memory. Results proved striking. Untreated Alzheimer’s mice and those receiving a scrambled control peptide showed poor performance, taking much longer to find the platform and spending less time in the correct area during memory tests. But mice treated with PHDP5 performed nearly as well as healthy animals. “We were thrilled to see that PHDP5 significantly rescued learning and memory deficits in the mice,” Dr. Chang said. “This success highlights the potential of targeting the dynamin-microtubule interaction as a therapeutic strategy for Alzheimer’s disease.” Double-blind tests, in which researchers did not know which treatment each mouse received, confirmed the results. PHDP5 did not enhance performance in healthy mice, ruling out a general cognitive boost. Instead, it appeared to rescue specifically the functions that Alzheimer’s had impaired.
byu/Hashirama4AP inEverythingScience
What PHDP5 Cannot Do
Important limitations apply to these findings. PHDP5 does not cure Alzheimer’s disease. It does not eliminate tau or prevent tangles from forming. Rather, it appears to work around the problem, keeping dynamin available for its normal job despite the presence of excess tau. For this workaround to succeed, treatment must begin early, before extensive damage has occurred. Once neurons die, no peptide can bring them back. PHDP5 offers a way to preserve function, not to resurrect what has been lost. Potential side effects also warrant attention. The interaction between dynamin and cell scaffolding serves purposes throughout the body, including in kidney function. Widespread suppression of this interaction could cause problems. Nasal delivery helps limit systemic exposure, but researchers will need to study long-term effects carefully.From Mice to Medicine
Grounds for Hope
Despite the long road ahead, researchers express cautious optimism. Dr. Chang points to recent history for encouragement. “The coronavirus vaccine showed us that treatments can be rapidly developed, without sacrificing scientific rigor or safety. We don’t expect this to go as quickly, but we know that governments, especially in Japan, want to address Alzheimer’s disease, which is affecting so many people. And now, we have learned that it is possible to effectively reverse cognitive decline if treated at an early stage.” Professor Emeritus Takahashi, who started the project before his retirement, has called for pharmaceutical companies to take the peptide through human clinical trials. He hopes that PHDP5 might reach patients without excessive delay and help rescue the cognitive symptoms that matter most to those living with the disease. For now, mice in an Okinawa laboratory continue to find hidden platforms and remember where they are. Whether humans will one day benefit from the same treatment remains an open question, but science has taken one more step toward answering it. Study citation: Chang, C.J., Taoufiq, Z., Yamada, H., et al. “The microtubule-dynamin binding inhibitor peptide PHDP5 rescues spatial learning and memory deficits in Alzheimer’s disease model mice.” Brain Research (2024). https://doi.org/10.1016/j.brainres.2024.14898Some of the links I post on this site are affiliate links. If you go through them to make a purchase, I will earn a small commission (at no additional cost to you). However, note that I’m recommending these products because of their quality and that I have good experience using them, not because of the commission to be made.

































 JOIN OVER
JOIN OVER
Comments